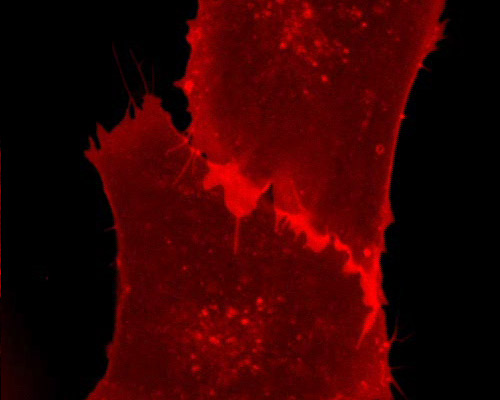
Grey Fox Lung Cells with mRuby-CAAX
Farnesyl Pyrophosphate synthase (FPPS) is a key intermediate enzyme in the isoprenoid biosynthesis pathway, which produces cholesterol and other polyisoprenes critical for membrane component synthesis and GTPase-based signal transduction. FPP is used by organisms in the biosynthesis of terpenoids, terpenes, and sterols.
In the selected digital video sequence, fox lung cells are shown expressing mRuby fused to proteins containing farnesyl groups. Protein isoprenylation is a post-translational modification involving the addition of a 15 carbon farnesyl group or a 20 carbon geranylgeranyl group to the C terminal of a protein. Isoprenylation is associated with a considerable increase in the hydrophobic characteristics of proteins and usually leads to increased membrane interactions. Examples of known prenylated proteins include nuclear lamins, Ras and Ras related small G proteins, and a subunit of trimeric G proteins.
The red fluorescent protein mRuby exhibits peak excitation and emission at 558 and 605 nanometers, respectively. Like eqFP611, the far-red fluorescent protein from which it was derived, mRuby exhibits a large Stokes shift. mRuby differs from its parent protein by 29 mutations and displays an outstanding resistance to denaturation in environments at extremes of both sides of the pH scale. The brightness of mRuby exceeds that of most other monomeric red fluorescent proteins developed to date.