The extent of mitochondrial presence in a cell is largely dependent on the metabolic needs of that cell. The size of the organelles ranges from 1 to 10 micrometers, which means mitochondria are large enough to be observed with a light microscope. Mitochondria were first observed during the 1800s, but most of the modern understanding of their function did not develop until the organelles could be successfully isolated intact, a process that was developed in the late 1940s.
Embryonic Rat Thoracic Aorta
Smooth Muscle Fibroblast Cells
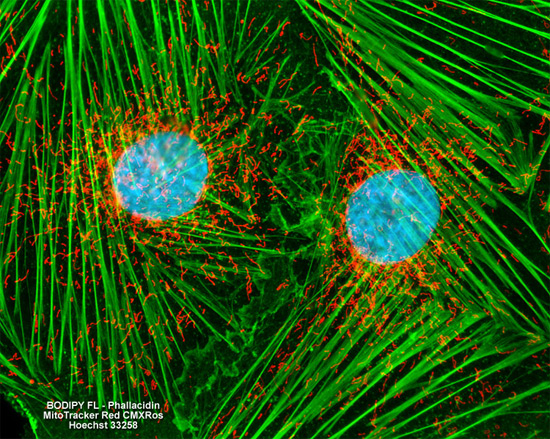
The popular triple fluorophore combination of MitoTracker Red CMXRos, BODIPY FL conjugated to phallacidin, and Hoechst 33258 was used to label the adherent log phase culture of rat thoracic aorta cells presented above for mitochondria, the filamentous actin network, and nuclear DNA. The cells were first treated with MitoTracker Red CMXRos in growth medium for one hour, washed and fixed with paraformaldehyde (prepared in growth medium), permeabilized, and blocked with bovine serum albumen. The cells were subsequently labeled with the conjugated phallotoxin and counterstained with the bisbenzimide reagent. Images were recorded in grayscale with a Hamamatsu ORCA AG camera system coupled to a ZEISS Axio Imager microscope equipped with bandpass emission fluorescence filter optical blocks provided by Chroma and Semrock. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles.